Evonik is introducing a new carbon-fiber reinforced PEEK filament, for use in 3D printed medical implants. This smart biomaterial can be processed in common extrusion-based 3D printing technologies such as fused filament fabrication (FFF). The specialty chemicals company will present the new product for the first time at coming next medical technology and 3D printing related trade shows.
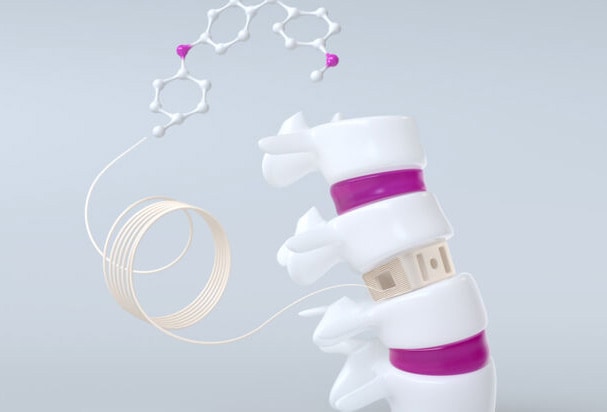

Evonik introduces the world’s first carbon-fiber reinforced PEEK filament for long-term 3D printed medical implants
Carbon-fiber reinforced PEEK for 3D printed medical implants
Dubbed VESTAKEEP® iC4612 3DF and VESTAKEEP® iC4620 3DF, the two available filaments feature 12% and 20% carbon fiber content, respectively. The two grades offer a choice of material depending on the required strength and flex properties of 3D printed implants such as bone plates and other reconstructive prostheses.
Evonik’s VESTAKEEP® iC4612 3DF and VESTAKEEP® iC4620 3DF offer great benefits from the strength from the high carbon-fiber content, matched with the ductility of its PEEK component. Additional product benefits include the ability to define the alignment of the carbon fibers during the 3D printing process, high bio-compatibility for metal allergy-patients, and the no x-rays artifacts.
Designing smart biomaterials for a life beyond limits
“By introducing the world’s first carbon-fiber reinforced PEEK filament for long-term medical implants, we continue to design biomaterials that open up new possibilities in today’s medical technology for patient-specific treatment,” says Marc Knebel, Head of Medical Systems at Evonik. “As passionate experts with decades of experience in polymer chemistry, we combine a unique set of competencies in materials science, manufacturing technologies and regulatory expertise to customers to accelerate time-to-market of new medical technologies for people’s lives beyond limits.”
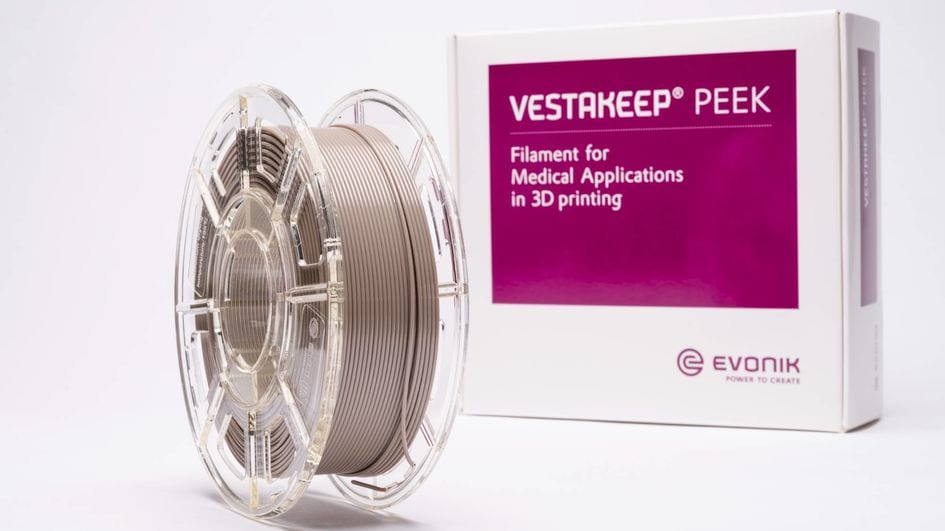
With a diameter of 1.75 mm, VESTAKEEP® iC4612 3DF and VESTAKEEP® iC4620 3DF are supplied on 500g and 1,000g spools that can be used directly in standard FFF/FDM 3D printers for PEEK materials. The filament is subjected to strict quality management for medical materials.
“No other application field showcases more the advantages of 3D printing, such as individualization or design freedom, than medical technology,” says Knebel. “In trauma applications, for instance, 3D printed solutions offer an enormous time advantage over traditionally manufactured medical devices. It is conceivable that patient-specific solutions can be manufactured within two or three days, significantly improving the recovery phase.”








